Dynamic consent in clinical genetic testing facilitates two-way communication, supporting informed patient autonomy for setting and modifying of consent preferences by patients over time, and these form the basis for dynamic downstream clinical and data management actions. It provides value to patients undergoing clinical genetic testing, healthcare professionals, organisations and broader society, yet despite benefits, its implementation and uptake is limited.
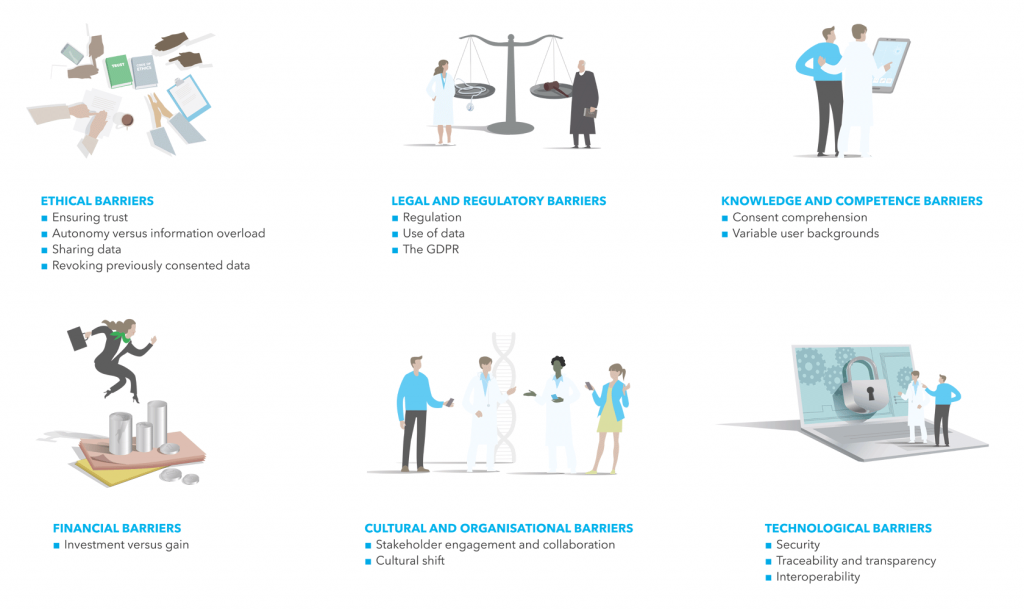
Through a literature review and semi-structured qualitative expert interviews, including with pioneering groups in dynamic consent, and supported by a survey of clinical genetics professionals on consent practices, DNV GL has mapped and identified six underlying categories of barriers that explain reasons for this, and discuss examples of approaches for overcoming these encountered during this work.
“Preparing the consent forms for our clinical genomic flagship project was challenging, where the forms were incredibly long and complex, not tailored to individual participant needs, and conveying information contained was labour intensive.”
Matilda Haas, Ph.D., Project Manager at CTRL, the Australian Genomics consent platform
The results of this work echoed and validated the perceived value that dynamic consent approaches may offer to patients, healthcare professionals, healthcare organisations and wider contexts. Even in cases where ongoing communication with patients is not required, future value may arise as new insight impacting care management is discovered, and as unforeseen factors support the transformation of genetic clinical care from a disconnected series of single interaction points to a more continuous care model.
In addition to the disruptive innovation that dynamic consent may bring to clinical genetics environments, it also has the potential to support paradigm shifts for medicine in other specialties. As more use cases develop where dynamic consent approaches can be applied, the barriers and challenges identified in this paper pinpoint several topics that should be considered prior to and as developments are underway. As a result, it is our hope that the findings in this paper can additionally be used to strengthen the discussions around dynamic consent applications in other settings.
SOURCE: https://www.dnv.com/research/precision-medicine/dynamic-consent-whitepaper.html
